RECHARGEABLE METAL ANODES
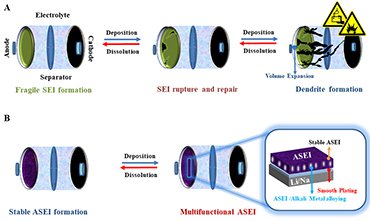 Realization of rechargeable batteries with alkali metal anodes is challenged by their high reactivity and dendritic growth. The high reactivity of alkali metals renders them intrinsically unstable against conventional battery electrolytes, promoting parasitic reactions on their surface, and, consequently, the formation of fragile solid electrolyte interphase (SEI). This leads to both undesirable electrolyte consumption and dendritic growth, which severely reduces battery performance.
However, by designing a multi-functional artificial SEI (ASEI) on the metal interphase, stabilization of alkali metals can be achieved. A properly surface engineered multifunctional ASEI not only protects the alkali metals, but also facilitates charge transfer and contributes to energy storage by alloying.
Realization of rechargeable batteries with alkali metal anodes is challenged by their high reactivity and dendritic growth. The high reactivity of alkali metals renders them intrinsically unstable against conventional battery electrolytes, promoting parasitic reactions on their surface, and, consequently, the formation of fragile solid electrolyte interphase (SEI). This leads to both undesirable electrolyte consumption and dendritic growth, which severely reduces battery performance.
However, by designing a multi-functional artificial SEI (ASEI) on the metal interphase, stabilization of alkali metals can be achieved. A properly surface engineered multifunctional ASEI not only protects the alkali metals, but also facilitates charge transfer and contributes to energy storage by alloying.

 © Copyright Noked Lab, all Right Reserved
© Copyright Noked Lab, all Right Reserved